Sunnyvale Lab Gives Details On How New Rapid Coronavirus Test Works
SUNNYVALE (KPIX 5) -- Officials with Sunnyvale-based company Cepheid on Sunday provided some explanation as to how its recently FDA-approved coronavirus test operates.
The company said it received FDA approval late Friday night to deploy its test -- designed to operate on the company's automated GeneXpert Systems -- that can get results in 45 minutes in a hospital laboratory.
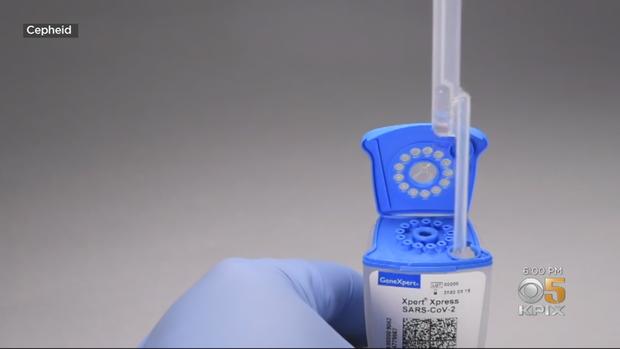
A healthcare worker takes a nasal swab from the patient and the sample is mixed in a tube. The liquid is then piped out into a cartridge.
The cartridge already has the necessary chemicals inside -- the so-called reagents missing from many of the COVID-19 tests when they were initially distributed -- so no more mixing is needed.
Technicians then snap the lid closed and drop it into the GeneXpert, a molecular testing station developed by Cepheid.
Everything stays contained within the cartridge. Using a series of pumps and siphons, the organisms are trapped on a filter.
The liquid reagent is then pumped to flush the trapped organisms, where a sonic horn breaks apart the organisms, forcing them to release DNA and other genetic material.
That genetic material is then rapidly heated and cooled while LED lights illuminate the sample.
If it glows bright with the right colors, that confirms the presence of the virus being tested for. The cartridge comes out and is disposed of.
While Cepheid declined on-camera interview requests this weekend, saying they are focusing their time and energy on shipping the tests this week, they released a pre-recorded statement from Dr. David Persing, the companys chief medical and technology officer.
And about 45 minutes later, you'll have the results, said Dr. Persing. I think it is transformative because this is the first time in the world that we've had this capability.
Persing noted that the speed of the test will have a major impact on treatment for the coronavirus.
We think this is an important tool to get rapid, actual results, said Persing. To let patients know if they're carriers, even if they're asymptomatic. To quarantine, and to know they're being quarantined for a reason. I'm very hopeful that we'll able to flatten the curve. Hopefully we can delay this enough so that we can allow the therapeutic approaches to have an impact.
FDA Commissioner Stephen Hahn released a statement that said, "Today marks an important step in expanding the availability of testing and, importantly, rapid results."
Cepheid says there are more than 23,000 Genexpert machines in operation worldwide. The new coronavirus test ships Friday with a planned rollout on March 30.