Updated 2024 COVID-19 vaccines arrive at Bay Area pharmacies
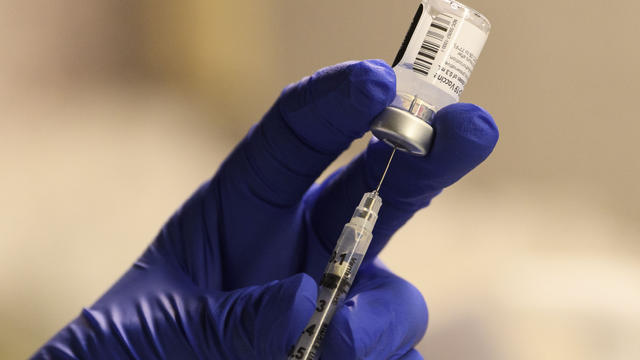
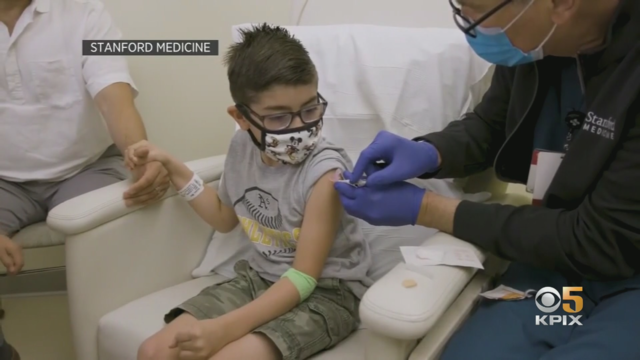
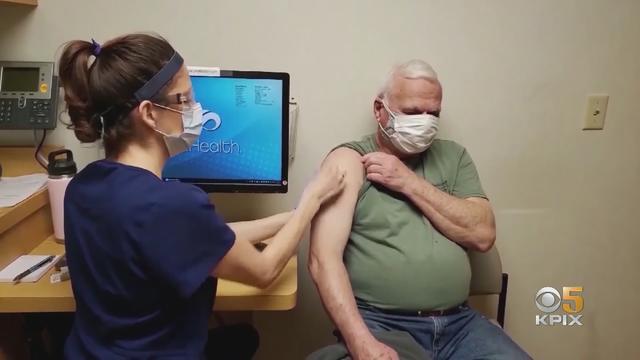
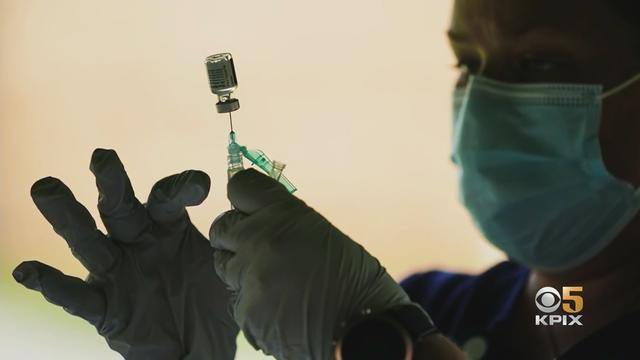
New COVID-19 vaccines have arrived at pharmacies in the Bay Area, designed to target the most recent FLiRT variants that have become dominant this summer. Sooji Nam reports. Website: http://kpix.com/ YouTube: http://www.youtube.com/CBSSanFrancisco Facebook: https://www.facebook.com/CBSSanFrancisco Instagram: https://www.instagram.com/kpixtv/ Twitter: https://twitter.com/KPIXtv